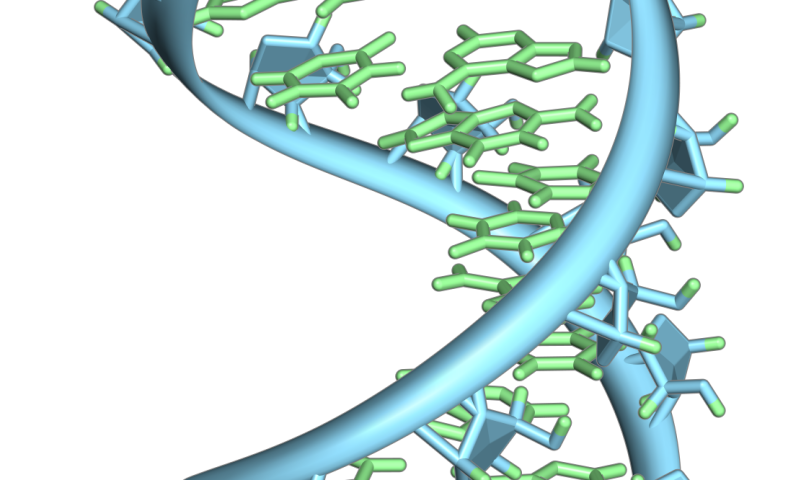
A hairpin loop from a pre-mRNA. Highlighted are the nucleobases (green) and the ribose-phosphate backbone (blue). Note that this is a single strand of RNA that folds back upon itself. Credit: Vossman/ Wikipedia
In a world-first, a team of Griffith University researchers has used an artificial intelligence method to better predict RNA secondary structures, with the hope it can be developed into a tool to better understand how RNAs are implicated in various diseases such as cancer.
Professor Yaoqi Zhou, Professor Kuldip Paliwal, Ph.D. student Jaswinder Singh and Dr. Jack Hanson from Griffith's Institute for Glycomics and Signal Processing Laboratory led the research, which has been published in Nature Communications.
In all forms of life, ribonucleic acid (RNA) is essential for the coding, decoding, regulation and expression of genes. RNA and DNA are among the four major macromolecules in lifeforms.
The team employed the use of deep learning—a subset of artificial intelligence used to create complex, numerical functions to approximate specific tasks automatically without explicit human instructions—to build a more accurate model of the relationship between RNA sequence and structure.
This advancement comes after more than a decade of stagnation in the performance of previous methods to predict RNA structures.
Professor Zhou hoped this new method would be useful for designing new RNA molecules with therapeutic potentials.
"Imagine if protein and RNA were two people, with protein standing in front of RNA—our focus is naturally on the protein," Professor Zhou said.
"Consequently, despite the fact that the number of proteins are outnumbered by the number of RNA by more than a factor of 10, we are clueless about what these RNAs are for in our human body.
"That's why we developed this tool: to provide some structural clues. Getting clues is very important because more and more RNAs are implicated in more diseases including various cancers.
"The most exciting aspect is that we can now better link the sequencing information with the structure. Our sequence is encoded in our genomes, but how they are related to their function through the structure is an unknown.
"Using this deep learning technique we can better link the sequence to the structure and have better clues as to what their function might be. Once we understand how the sequence encodes the structure and therefore function, we can design the RNA to do it for a particular purpose, such as new drugs or molecular sensors."
In order to develop the method, the team had to expand on existing data sets for known RNA structures by sourcing approximated computational data, then refine the automated training method with the exact data.
Professor Paliwal said only having access to less than 250 non-redundant known RNA structures among about 30 million unknown was a challenge that only the use of their deep learning method could address.
"Deep learning was used in this research to model the fundamental relationship between an RNA's nucleotide sequence and the pairing of these nucleotide bases in its functional structure," Professor Paliwal said.
"This is a very complex function as, theoretically, a nucleotide can be paired with any other base within the RNA, so it is the job of the deep learning neural network to find out which nucleotides are paired together.
"Making matters even more complex is that these algorithms have to be general and work for the millions of unique RNA sequences.
"Before our work, most of the previous studies had relied on comparative schemes based on RNA biological families or handcrafted scoring algorithms based on statistics. These methods can somewhat model the incredibly intricate function linking an RNA's nucleotide sequence to its paired structure, but had reached a stagnant performance ceiling of about 80% accuracy for basepair predictions.
"Using deep learning, we were able to overcome all of these shortcomings to provide a blanket solution for all RNA structures while simultaneously breaking the performance ceiling which had existed for more than a decade, attaining a basepair accuracy of 93%."
The team said the use of deep learning for the prediction of RNA basepairs was a feasible tool and a world first, and achieved superior performance in almost every facet compared to previous attempts.
Founder and Director of the Institute for Glycomics Professor Mark von Itzstein AO said the finding "opened up avenues for future research into this problem from other computational research groups, while providing a more accurate tool for experimental laboratories working in the fields such as biomedicine, drug discovery, and molecular biology."
The research "RNA secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning' has been published in Nature Communications.
More information:
Jaswinder Singh et al. RNA secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning, Nature Communications (2019). DOI: 10.1038/s41467-019-13395-9
Citation:
Researchers use deep learning method to delve into predicting RNA structures in world first (2019, November 27)
retrieved 27 November 2019
from https://phys.org/news/2019-11-deep-method-delve-rna-world.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
#Biology | https://sciencespies.com/biology/researchers-use-deep-learning-method-to-delve-into-predicting-rna-structures-in-world-first/
No comments:
Post a Comment